46人活活烧死…欧洲10年最惨车祸何以酿成?
文章来源: 联合新闻网
11/24/2021
“每次重大死伤都只有司机『被负责』?这只是交通设计者的掩耳盗铃。”欧盟国家保加利亚23日凌晨2点发生一起死伤极为惨重的重大交通事故,一辆来自北马其顿的旅行团巴士,在恶名昭彰的斯特鲁马高速公路(A3 Struma)自撞护栏后”爆炸”,包含驾驶与十多名幼童在内,全车至少46人被困在车上活活烧死——这不仅是保加利亚史上最严重的交通事故,也是近11年来,欧洲记录死伤最惨烈的车祸惨案。
消息传开后,不仅全欧震惊,保加利亚与北马其顿也随即颁布”国殇日”,但极为骇人的事故证词与现场说法,却随着时间的前进而逐一矛盾曝光。在最初一波的说法里,官方发现肇事的巴士与驾驶并没有取得”跨国运输执照”,质疑大巴司机极可能是”疲劳驾驶”或”危险超速”才会害死大批旅客、酿成死亡惨剧。
但保加利亚的交通安全NGO与运安专家们却质疑:启用仅15年的斯特鲁马高速公路,本来就是施工质量差、道路设计极为不良而屡传事故的”死亡公路”。但作为欧盟交通死亡率最高的保加利亚,不仅完全无视专家NGO的历年警告,最终更可能默许不当设计”诱发”了这回让46人被活活烧死的残酷悲剧?
Nov 23, 2021
‘TRAGIC Bulgarian Bus INFERNO Claims 46 Lives’
A deadly bus crash on a frozen Bulgarian road has claimed the lives of at least 46 people.
Children were among the victims, while seven people were taken to hospital.
The vehicle, with 52 people on board, crashed around 2am local time.
发生在斯特鲁马公路的死亡惨案,是在11月23日凌晨2点,一辆北马其顿籍的”跨国旅行团巴士”,正结束土耳其的观光行程,载着至少53名北马其顿人,途经保加利亚”夜车返家”。
官方表示,失事的巴士是北马其顿公司”Besa Trans国际旅行社”的4辆出团包车之一,该公司营业资历超过20年,专营北马其顿团客前往土耳其观光购物的平价旅行团——事发当时的车祸大巴,则是周一下午从土耳其伊斯坦堡返航,途经保加利亚首都索非亚,接着走上南向的斯特鲁马高速公路,准备趁夜衔接驶回北马其顿首都斯科普里。
周二凌晨,大巴车队先是在索非亚近郊的休息站加油休息,准备在上完厕所、吃完消夜后,全团一路拚夜车,好在周二上午能够睡醒到家。不料当车队重新出发、走上斯特鲁马公路山区才半个多小时,车队垫后的第4辆大巴却在23日凌晨2点左右与前方团队失联。
Nov 23, 2021
一开始,向前奔驰的3辆巴士都没发现”友车落队”,一直到公路警察紧急通知后众人才知大事不好——原来掉队的第4辆大巴,在斯特马鲁公路南下31~32公里的路段出了严重车祸,
“整辆巴士不仅车祸起火,更马上发生爆炸——除了7名后座乘客侥幸『徒手破窗』之外,包括司机在内的『所有人』都被活活烧死在车上。”
巴士爆炸之后,保加利亚公路警察与大批警消虽然很快赶达,但过于汹涌的爆炸火势却在极短时间内把全车烧得只剩骨架,除了自行幸运逃生的7名乘客外,其他人全员被烧死在车内、根本来不及逃生。
保加利亚公路警察局表示:根据现场的初步调查,巴士车祸前正行驶在长下坡的大转弯之前,但司机疑似不熟路线或”打瞌睡恍神”,因此失误开上右线道的避车弯,右前车身撞上了槽化线护栏,接着高速行驶的巴士才会在公路上失去控制。失控的撞车巴士,随即往左侧高速横移40多公尺,然后直接撞破左侧护栏,接着全车卡在安全岛上”马上爆炸”。
巴士之所以爆炸起火,目前粗判的原因可能是第一次撞车(右)所导致的油箱破损,并被第二次撞车(左)的火花引燃,因此才会在极短时间内由中前段开始陷入”全车火海”,除了后座几名乘客极为侥幸地”赤手打破右后排车窗”跳车逃生,其他乘客都在车祸堆栈与惊恐中,直接被烈焰吞噬。
现场检察官表示,虽然在撞车之后,巴士的逃生门与前门仍能开启、没有变形,但车身起火所导致的爆炸火势来得过猛又快,
“在灾后现场,我们看到大量遗体堆栈在车内前门通道——大家不是打不开出口,是大火来得太快,众人根本接近不了门。”
大量遗体堆栈的惨况,也严重影响了灾后的鉴识与罹难者身份通知。许多北马其顿家庭一直到23日早上看到新闻快报后才知道”旅行团出事”,但无论是主办的Besa Trans、北马其顿外交部或保加利亚政府,却都无法确认死难乘客名单,甚至连安排统一联络窗口都有困难。不少在连续打电话联络亲属未果后,直接开车跨国赶到索非亚的救护医院,希望直接在灾后现场确认”失联亲友是生是死”,但复杂的跨国外交交涉与辨识问题,却让这些焦急亲属不得其门而入,进而在北马其顿社会引发了很大的恐慌焦虑与愤怒民怨。
但对于北马其顿方面的不满,主导调查的保加利亚内政部长则无奈地表示:这起惨重事故的辨识确实难度极高,因为在开上斯特鲁马公路前,4辆旅行团巴士曾在索非亚近郊的加油站”休整换位”——因为不同团员的返家目的地不一样,数百名乘客才会在此复杂换位,这导致每车的旅客人数、姓名名单需要重新连络比对,官方才迟迟无法公布死伤名单、甚至连确认受难总人数都有困难。
除此之外,火场辨识的难度也让保加利亚鉴识组快不起来。保国内政部长强调:
“死在巴士的罹难者,不仅全都挤在一起——猛烈火势更把堆栈的遗体全都烧成了焦灰…现场根本无法外显辨识死者的身份与数量。”
正因破坏非常严重,保国警方也只能在重新整理旅行团名单的同时,连夜请求北马其顿本国刑警协助鉴识。而跨国刑警合作,与大量身份未明的焦尸、遗骨辨识,也让这起悲伤的重大惨案显得更为考验且残酷。
截至11月24日清晨为止,斯特鲁马高速公路惨案据传已知”至少47死”,全车生还7人中又有5人重伤无法应讯——全案不仅是保加利亚史上死伤最严重的车祸事故,也是欧洲全大陆近11年来最为严重的”公路意外”。
巴士惨案传开后,举国低迷的北马其顿即宣布自周三开始”国殇3日”;尽管已知所有死伤者全都是北马其顿籍的外国人,地主保加利亚也同样全国降半旗、国殇1天。但后续的究责与调查进度,却也显示了全案的错综复杂与诿过纠结。
事发后的第一时间,保加利亚检警就向媒体透露,认为全案”要不是巴士养护不当的机械故障”,要不就是”死掉的司机疲劳驾驶或危险超速”。但当前线证词回传后,事情的样态却开始变得有点吊诡——因为在车祸发生的20分钟前,几辆巴士才在索非亚的加油站休息,就算深夜开在山区的长途高速公路上确实容易疲倦,但要在短短20分钟内睡着或再陷极端疲惫?在目前调查中似乎显得有些不合常理。
事实上,保加利亚政府的肇事论点,在第一时间里就造到自已国内的运安专家痛批质疑。像是保加利亚国内NGO”道路安全研究中心”的道安专家鲁西诺娃(Diana Rusinova),23日就公开指控:
保加利亚交通部应该负最大责任,因为斯特鲁马高速公路的”瑕疵设计”问题,过往早就是屡屡酿成死亡车祸、公民团体屡屡纠举却没人处理的著名”死亡公路”。
落成仅15年的斯特鲁马高速公路,是保加利亚连结欧盟交通的重要建设,其主要衔接索非亚通往希腊的南北向干道,是相当繁忙与重要的转运中轴。
然而这条由法国统包建设、北马其顿营造公司分包施工的高速公路,素以施工质量极差、道路设计不良而臭名昭彰。其最主要被用路者诟病的问题,是路面质量极差,在途经多雨多雾的山区路段——像是本次巴士车祸的地点即为代表一例——偷工减料的不平公路,极容易打滑失事。全线也有极多设计不良的危险山区陡坡弯道,照明、道路监控系统、路标指示也都严重不足或混乱,甚至连一般的公路地面划线、分隔线,都能偷工减料而模糊不清。
“每次个案都可以归咎为危险驾驶,但一次两次过后,悲剧还是不断发生,这是因为道路设计真的很烂、工程质量真的很烂…我们总不可能无止尽地把每一次车祸,都把罪责推给司机!”
鲁西诺娃以本回事故路段为例,指巴士在遭遇大弯道前没有充足的警告与指示,因此才会在外侧右线道一转弯就撞上槽化线栅栏,从中根本没有足够的道路预警与缓冲空间,更何况是在视线不佳的雨夜凌晨,这对一般本国用路人都有危险了,更何况是那些长途驾驶疲惫的欧洲跨国线司机呢?
事实上,保加利亚的道路质量与交通事故率,一直是欧盟各国中的垫底最低。尽管欧盟每年会拨给保加利亚、罗马尼亚等后进的东欧成员国大笔建设预算,作为升级公路基础建设与道路安全机制的发展资金。但长年的投资与开发却仍难以回避设计不良与工程贪腐的基层问题。
不过对于运安专家的质疑与控诉,保加利亚检方却是不以为然地强调,事发路段的公路状况非常不错,没有路面破损、不平或湿滑的问题,”后续调查才正要开始,不必要过度揣测”。

北美法律公益讲座安排
时间:周二到周五 晚间
5:30-7:00(西部)
8:30-9:30(东部)
第二天西部时间早上9:00重播
周二: 如何准备遗嘱文件(遗嘱workshop)
周三: 数据泄露和个人身份保护&事业机会说明会
周四: 移民和留学生常见法律问题
周五:小企业企业常见法律问题&事业机会说明会
Zoom 6045004698,
密码:进群获取

默沙东抗疫口服药可降低50%住院或死亡风险
文 / 麦可欣
10/02/2021
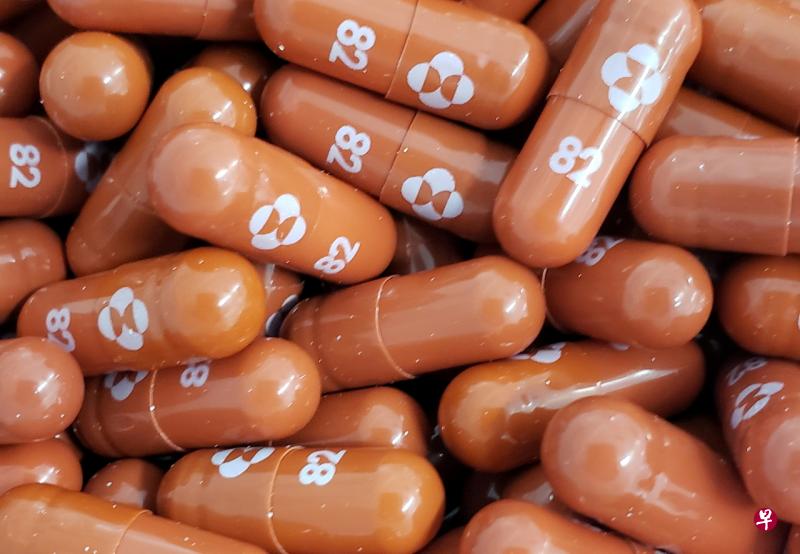
(早报讯)美国默沙东药厂(Merck & Co Inc)周五(10月1日)公布,研发中的冠病实验口服药“molnupiravir”,可使重症高风险患者的住院或死亡率降低约50%。默沙东表示,将向美国当局申请紧急授权使用,一旦获批准,将是全球首款抗冠病口服药。
默沙东药厂发表声明说,:“根据期中分析,服用molnupiravir的染疫患者中,至29天有7.3%住院;而服用安慰剂的患者,至29天有14.1%住院或死亡。”
默沙东药厂计划针对这个口服药尽快向美国提出紧急使用授权,并且向全球监管机关递交申请。由于临床实验结果证实有疗效,在外部监测人员建议下,第三期临床试验提早喊停。
Merck says its new Covid pill reduces the risk of hospitalization, death by half for some patients
By Chloe Taylor
10/02/2021
KEY POINTS
- A phase 3 trial of Merck and Ridgeback Biotherapeutics’ oral antiviral treatment molnupiravir showed it reduced the risk of hospitalization or death by around 50% in Covid patients.
- Merck plans to seek emergency use authorization in the U.S. and submit marketing applications to other global drug regulators.
- If authorized by regulatory bodies, molnupiravir could be the first oral antiviral medicine for Covid.
- “The company, when they briefed us last night, had mentioned that they will be submitting their data to the FDA imminently,” White House chief medical advisor Dr. Anthony Fauci said at a Covid briefing Friday.
Merck and Ridgeback Biotherapeutics said Friday they’ve developed a drug that reduces the risk of hospitalization or death by around 50% for patients with mild or moderate cases of Covid.
The companies plan to seek emergency authorization for the antiviral Covid treatment after the medicine showed “compelling results” in clinical trials.
The drug, molnupiravir, is administered orally and works by inhibiting the replication of the coronavirus inside the body.
An interim analysis of a phase 3 study found that 7.3% of patients treated with molnupiravir were hospitalized within 29 days. Of the patients who received a placebo, 14.1% were hospitalized or died by day 29. No deaths were reported in patients who were given molnupiravir within the 29-day period, while eight deaths were reported in placebo-treated patients.
“The news of the efficacy of this particular antiviral is obviously very good news,” White House chief medical advisor Dr. Anthony Fauci said at a Covid briefing Friday. “The company, when they briefed us last night, had mentioned that they will be submitting their data to the FDA imminently.”
“The FDA will look at the data and in their usual, very efficient and effective way, will examine the data as quickly as they possibly can, and then it will be taken from there,” Fauci said.
All 775 trial participants had laboratory-confirmed symptomatic Covid-19 and were randomly given molnupiravir or a placebo within five days of symptoms.
Every participant was unvaccinated and had at least one underlying factor that put them at greater risk of developing a more severe case of the virus. The most common risk factors included obesity, being over age 60 and having diabetes or heart disease.
The phase 3 part of the trial was conducted at more than 170 sites, in countries including the U.S., Brazil, Italy, Japan, South Africa, Taiwan and Guatemala.
Molnupiravir’s efficacy was not affected by the timing of symptom onset or patients’ underlying risk factors, the study showed. It also proved to be consistently effective in treating all variants of Covid, including the widely dominant and highly transmissible delta strain.
Adverse events were comparable in the molnupiravir and placebo groups, with around 10% reporting adverse events. Just 1.3% of the molnupiravir group discontinued therapy due to an adverse event — less than the 3.4% of the placebo group who did so.
Recruitment into the study is being stopped early due to the positive results, at the recommendation of an independent Data Monitoring Committee and in consultation with the U.S. Food and Drug Administration.
Merck is also testing molnupiravir in a separate global phase 3 study to evaluate its efficacy in preventing the spread of Covid within households.
‘Profound impact’
Robert M. Davis, CEO and president of Merck, said in a press release Friday that the company would do everything it can to bring molnupiravir to patients as quickly as possible.
“With these compelling results, we are optimistic that molnupiravir can become an important medicine as part of the global efforts to fight the pandemic,” he said.
Ridgeback Biotherapeutics CEO Wendy Holman added: “With the virus continuing to circulate widely, and because therapeutic options currently available are infused or require access to a healthcare facility, antiviral treatments that can be taken at home to keep people with Covid-19 out of the hospital are critically needed.”
“We are very encouraged by the results from the interim analysis and hope molnupiravir, if authorized for use, can make a profound impact in controlling the pandemic,” she said.
Emergency use authorization
Merck said Friday it plans to seek emergency use authorization for the drug in the U.S. as soon as possible. The company also plans to submit marketing applications to other international drug regulators.
If authorized by regulatory bodies, molnupiravir could be the first oral antiviral medicine for Covid. Antiviral treatments now in use, such as remdesivir, are administered intravenously.
Merck has already begun producing molnupiravir. The pharmaceutical giant expects to produce 10 million courses of treatment by the end of 2021, and more doses in 2022.
The company agreed earlier this year to supply the U.S. with around 1.7 million courses of molnupiravir if it receives emergency use authorization or full approval from the FDA. The federal government also has the option to purchase additional doses if the drug is approved, White House coronavirus response coordinator Jeff Zients said at Friday’s briefing.
Merck has also entered supply and purchase agreements for the drug with other governments — pending regulatory authorization — and is in discussions with other governments about the supply of molnupiravir.
The company said it plans to implement a tiered pricing approach based on World Bank country income criteria to ensure molnupiravir can be accessed globally. Merck previously announced that it had entered into nonexclusive voluntary licensing agreements for molnupiravir with generic manufacturers, a move intended to assist low and middle-income countries in gaining access to the treatment. Those agreements are also pending approvals or emergency authorization by local regulators.
Profit share
Ridgeback received an upfront payment from Merck as part of the companies’ development of molnupiravir. The company is also eligible to receive contingent payments depending on developmental and regulatory approval milestones.
Profits arising from the collaboration will be split between Merck and Ridgeback equally.
Supreme Court Justice Brett Kavanaugh tests positive for COVID-19
Kavanaugh shows no symptoms, is fully vaccinated, court says
By Houston Keene | Fox News
10/01/2021
Supreme Court Justice Brett Kavanaugh tested positive for COVID-19, the court announced Friday, noting that he’s fully vaccinated and showed no symptoms.
Kavanaugh learned of the positive test Thursday evening, ahead of Justice Amy Coney Barrett’s ceremonial investiture Friday morning, according to a news release from the court.

The release added that Kavanaugh’s wife and daughters, all fully vaccinated, tested negative.
“On Thursday, per the Court’s regular testing protocols, Justice Kavanaugh had a routine Covid test ahead of Justice Barrett’s investiture on Friday,” the release said. “On Thursday evening, Justice Kavanaugh was informed that he had tested positive for Covid-19.”
“He has no symptoms and has been fully vaccinated since January. Per current Court testing protocols, all of the Justices were tested Monday morning prior to conference, and all tested negative, including Justice Kavanaugh,” it continued.
Barrett and Kavanaugh will be sitting at opposite ends of the table when oral arguments resume in the Supreme Court on Monday.
Kavanaugh ran in the Capital Challenge Road Race on Wednesday, with his team winning the three-mile race’s judicial division with a 25 minute time.
Several lawmakers, judges and members of the media also participated in the race. Fox News’ Sandra Smith placed first in the Electronic Journalist – Female category while Fox News took first in the Electronic Media category.
COVID-19 breakthrough cases have hit Capitol Hill recently. Fully vaccinated South Carolina Republicans Sen. Lindsey Graham and Rep. Ralph Norman tested positive for the virus in August.
As of Friday, the U.S. has 3.7 million COVID-19 cases and almost 52,000 deaths reported within the last 28 days, according to Johns Hopkins University’s COVID-19 tracker.
1 in 500 US residents has died of Covid-19
By CNN Wire
9/15/2021
(CNN) — The United States has reached another grim milestone in its fight against the devastating Covid-19 pandemic: 1 in 500 Americans have died from coronavirus since the nation’s first reported infection.
As of Tuesday night, 663,913 people in the US have died of Covid-19, according to Johns Hopkins University data. According to the US Census Bureau, the US population as of April 2020 was 331.4 million.
It’s a sobering toll that comes as hospitals in the US are struggling to keep up with the volume of patients and more children are grappling with the virus. In hopes of managing the spread and preventing more unnecessary deaths, officials are implementing mandates for vaccinations in workplaces and masking in schools.
They are fighting against a sharp upward trend in cases and deaths: The US is reporting a more than 30% increase in average daily cases and a near tripling of average daily deaths over the past month, according to data from the US Centers for Disease Control and Prevention.
But with only 54% of the population fully vaccinated, the rate of people initiating vaccinations each day has declined over the past month.
Health experts have hailed vaccinations as the best source of protection against the virus, noting that the majority of people hospitalized with and killed by Covid-19 are unvaccinated. In Pennsylvania, from January 1 to September 7, 97% of the state’s Covid-19 deaths were among unvaccinated people, Pennsylvania’s acting secretary of health said Tuesday.
Another layer of strong protection, experts say, is masking.
The CDC recommends people — even those fully vaccinated — wear masks indoors in areas with substantial or high community transmission. More than 99% of the population lives in a county with one of those designations.
In Ohio, where children’s hospitals are overwhelmed with Covid-19 and respiratory cases, Gov. Mike DeWine is encouraging schools to issue mask mandates since the state legislature has told him it would overturn any mandate he issued.
“Reasonable people may disagree about a lot, but we can all agree that we must keep our children in the classroom so they don’t fall behind and so their parents can go to work and not take time off to watch their kids at home,” DeWine said.
The combination of masks and vaccinations is the way to keep children in school, Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told CNN Tuesday.
“If you surround the kids with vaccinated people and you have everybody wear a mask, you can get a situation where the children will be relatively safe in school,” Fauci told CNN’s Jake Tapper.
Fight brewing over vaccine mandates
In the effort to manage the spread of the virus, many officials and experts have promoted vaccine mandates — but others are opposing such measures.
New York issued an order in August requiring all health care workers be vaccinated against Covid-19 by September 27. But on Monday, 17 Catholic and Baptist medical professionals filed a federal complaint seeking to prevent the state from enforcing the mandate, saying they oppose getting the vaccine for religious reasons.
On Tuesday, a federal judge issued a restraining order temporarily suspending New York state from enforcing its vaccine mandate if health care workers claim a religious exemption.
Because the mandate does not require health care workers to receive their first dose of the vaccine until September 27, the judge’s order states the temporary restraining order “does not, as a practical matter, go into effect until that date.”
A hearing is scheduled for September 28.
After the ruling, New York Gov. Kathy Hochul’s press secretary, Hazel Crampton-Hays said in a statement that the governor is considering all legal options.
“Governor Hochul is doing everything in her power to protect New Yorkers and combat the Delta variant by increasing vaccine rates across the State,” Crampton-Hays said.
In Los Angeles, despite a mandate that all city employees be inoculated against the virus, nearly a quarter of the police force is seeking an exemption, according to Mayor Eric Garcetti’s office. Those who are not vaccinated will be required to show evidence of weekly testing and a negative COVID result if regularly reporting to work.
By November 1, Nevada workers who serve “vulnerable populations” must show proof of vaccination under a new emergency regulation passed Tuesday.
New hires must have at least one dose by their start date and must follow through on the required vaccination schedule to remain employed. Workers are allowed to ask for a medical or religious exemption.
Booster meeting won’t be a slam dunk
On Friday, the US Food and Drug Administration (FDA) will meet to discuss whether most Americans need a booster of their Covid-19 vaccine.
Unlike other meetings to discuss the vaccine, this one, with requests from Pfizer to authorize a third dose for most people, won’t be a slam dunk.
“This will be much messier than in December,” said Dr. William Schaffner, an infectious disease specialist at Vanderbilt University. The FDA committee was quick to recommend authorization of vaccines made by Pfizer and rival Moderna last December.
When the FDA’s Vaccines and Related Biological Products Advisory Committee meets Friday, it will be presented with dueling data, some of it suggesting there’s a need for boosters, but other pieces of data suggesting there is no such need.
Three separate articles published last week in the CDC’s Morbidity and Mortality Weekly Report suggest that we don’t need boosters.
On the other hand, an Israeli study found that over time, the vaccines’ power to keep people from getting very sick with Covid-19 diminished. Looking at illnesses in the second half of July, that study found that those who’d received their second dose of Pfizer’s vaccine in March were 70% more protected against severe disease than those who received the second shot in January.
President Joe Biden announced plans last month to begin administering booster doses next week. While she wouldn’t say directly if that date would be met, CDC Director Rochelle Walensky said Tuesday she is hopeful about the timeline to get doses administered.
If the booster does get approved, experts will still have to wait and see how much protection is added by the third dose.
“I would hope that that would sustain us for an extended period of time, but I don’t know that right now,” Fauci said. “We’re just going to have to do the boost, and then follow people long enough to determine what the durability of that protection is.”




