港专家:香港没条件与冠病病毒共存
10/24/2021

香港政府专家顾问、中大呼吸系统科讲座教授许树昌说,香港整体的冠病疫苗接种率目前仅约六成,加上长者的接种率低,不但难达到“绝对清零”,目前也没有条件采取“与病毒共存”的策略。
综合网媒“香港01”、香港电台、文汇网报道,许树昌今早在商台节目上说,香港整体疫苗接种率大约六成,但长者接种率低;此外,有约四成接种者选择注射中国大陆的科兴疫苗,有数据显示其抗体于接种两针六至八个月后,降至低水平。因此,他认为香港没有条件采取“与病毒共存”的策略。
许树昌也说,英国等国家决定采取“与病毒共存”的策略后,冠病确诊个案严重暴发,加上冬季即将来临,他预估未来将会有更多变量。
许树昌还提到,如果民众首两剂接种灭活平台的疫苗,第三针转为接种另一平台的疫苗、例如核酸疫苗会较好,因为可刺激产生更多抗体。他相信,可能每一至两年,民众或要接种冠病疫苗加强剂一次。
康希诺:两剂灭活加强一剂吸入 抗体最高升300倍
10/17/2021
中国康希诺生物公司发布吸入式冠病疫苗研究成果,临床数据显示,两剂灭活加强一剂吸入,中和抗体水平最高可上升300倍。
据澎湃新闻报道,康希诺生物股份公司首席科学官朱涛在第21届中国生物制品年会期间进行了《腺病毒载体疫苗研发历程——如何与病毒赛跑》的主题演讲。
在介绍吸入式腺病毒载体新冠疫苗克威莎加强免疫的临床数据解读时,朱涛提到,接种两剂灭活疫苗后6个月,序贯加强一剂克威莎吸入剂型,中和抗体水平相比加强之前升高250-300倍;若同源加强一剂灭活疫苗,中和抗体水平相比加强前升高30倍。
康希诺生物在其微信公众号上发文称,以吸入型腺病毒载体新冠疫苗进行异源序贯加强,相较第三针灭活疫苗加强更有优势,抗体升高倍数,约是以一剂灭活疫苗加强的7-8倍。另外,吸入序贯免疫可获得针对原型株、Alpha、Beta、Gamma、Delta株的高效中和抗体。
文章还提到,数据显示,接种两针次灭活新冠疫苗后,用不同疫苗加强免疫,腺病毒载体疫苗加强效果最好,mRNA疫苗效果接近腺病毒载体,灭活或者亚单位疫苗加强显著低于腺病毒和mRNA疫苗,其中和抗体水平差异接近10倍。
对于雾化吸入式疫苗的有效性,12日晚间,江苏省疾控中心副主任、临床疫苗专家朱凤才在一次分享中也提到,雾化吸入可获得粘膜免疫、细胞免疫、体液免疫“三重保护”,在病毒入侵位置预防感染和阻断传播,保护自己也保护他人。雾化吸入新冠疫苗在加强、序贯免疫效果显著,适用于风险人群的大规模加强免疫。
Inhalable vector-based COVID-19 vaccine increases antibodies 300-fold, better than mRNA & inactivated type as booster
By Global Times
10/17/2021
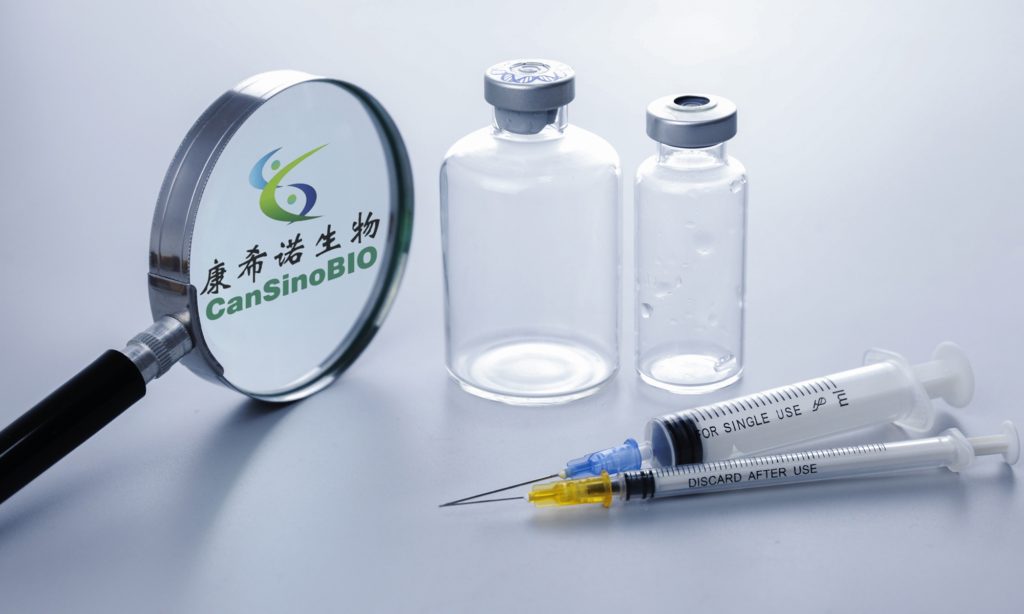
According to latest lab studies, China’s first aerosolized COVID-19 vaccine has showed an increase of 250- to 300-fold in neutralizing antibody levels after two rounds of inactivated vaccine administration, proving greater efficacy in using a combination of different types of vaccines to enhance effects.
Taking the CanSinoBIO’s aerosolized inhaled adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) as a booster shot after completing two doses of inactivated vaccine shots for half a year is proven to be safe and significantly more immunogenic than taking an inactivated vaccine as a booster, said Zhu Tao, co-founder and chief scientific officer of the CanSinoBIO, at a recent industrial conference.
In contrast, neutralizing antibodies can increase by only 30 times if taking an inactivated vaccine as a booster after two inactivated vaccine shots.
Zhu cited a previous study conducted in Turkey whose data showed a booster dose of the mRNA vaccine widely used in Western countries for people administered along with two inactivated doses can increase the neutralizing antibodies by about 25 times as compared with a booster dose of the inactivated vaccine again.
These clinical trials suggested that a heterologous prime-boost regimen can increase the breadth, intensity and duration of the immune response, more than a homogeneous booster shot.
US researchers previously conducted clinical trials on a heterologous booster shot regimen with three approved vaccines – one adenovirus-based vaccine by Johnson & Johnson and two mRNA vaccines by Moderna and Pfizer. The results similarly showed that heterologous enhanced immunity had obvious advantages.
The study showed that boosting with any of the three vaccines currently licensed or authorized for emergency use in the US will stimulate an anamnestic response in persons who previously received any of the primary series of any of these vaccines. Homologous boosters increased neutralizing antibody titers 4.2- to 20-fold whereas heterologous boosters increased titers 6.2- to 76-fold, said the research report published on medRvix on October 13.
So far, at least 13 provinces and regions in China, such as East China’s Anhui and Fujian provinces, and Central China’s Hubei Province, have initiated programs to enhance residents’ immunity against COVID-19.
The latest studies proved that taking the approved Chinese vaccines – inactivated and vector-based ones – in heterologous a booster regime is expectedly the most effective one among all current world-wide regimens using the mRNA vaccine as a booster.
Global Times

Tel: 551-580-4856 | Email: F.WINNIE.S@GMAIL.COM
诚招美国和加拿大法律服务代理
因公司发展需要,诚招美国和加拿大法律服务代理。
要求:
懂英语、或西班牙语、或法语。
能合法工作有社安号或工号。
无需改行, 可以兼职。
大学生和有销售经验优先考虑。
自雇生意公司发美国报税1099,加拿大T4A
有意了解详情, 请扫码加微信, 非诚勿扰!
阿斯利康抗体鸡尾酒药物后期研究取得成功
文 / 陈慧璋
10/11/2021

(早报讯)英国生物制药公司阿斯利康周一(11日)说,其试验性冠病抗体鸡尾酒药物在一项后期研究中成功减少非住院患者演变成重症或死亡。
路透社报道,出现七天或少于七天症状的冠病患者使用这款名为AZD7442的抗体鸡尾酒药物后,演变成重症或死亡的风险降低了50%,这符合了这项临床试验的目标。
阿斯利康研发部执行副总裁潘加洛斯说:“使用我们这款抗体药物对冠病病毒进行早期干预,可以显著降低病情恶化的风险,并可为患者持续提供六个月以上的保护。”
另外,阿斯利康也计划把这款抗体鸡尾酒药物开发为适用于无法对冠病疫苗产生足够免疫力的人的冠病药物。
阿斯利康上周已就这款抗体鸡尾酒药物作为冠病预防性药物,向美国药物监管当局申请紧急使用授权。
日本调查:接种首剂阿斯利康疫苗 66%出现倦怠感
10/03/2021
(早报讯)日本卫生、劳动与福利部研究小组日前公布的调查结果显示,在首次接种英国阿斯利康冠病疫苗的每三人当中,大约有两人会出现倦怠感。这是日本政府首次公布有关阿斯利康疫苗不良反应的调查结果。
新华社引述日本广播协会电视台(NHK)报道,研究小组对今年8月以来在日本八所医疗机构接种第一剂阿斯利康疫苗的179人展开调查。
结果显示,大约66%的人出现倦怠感;51%的人出现头痛症状;47%的人则发烧,体温达37.5摄氏度以上。越年轻,越容易出现上述不良反应,且多见于接种后的第二天和第三天。
研究小组负责人、顺天堂大学医学系客座教授伊澄信表示,其他冠病疫苗在第二剂接种后更易出现症状,而调查数据显示,阿斯利康疫苗在第一剂接种后更易出现不良反应。
据NHK报道,截至当地时间星期六(10月2日)傍晚6时30分(新加坡时间晚上7时30分),日本新增1246起冠病确诊病例,累计确诊病例达170万3880起;新增死亡病例则有32起,累计1万7719起死例。
2 out of 3 feel fatigue after 1st AstraZeneca jab
10/03/2021
Japanese researchers have found that two out of three people experienced fatigue after their first shot of the AstraZeneca coronavirus vaccine.
A research team of the health ministry analyzed the symptoms of 179 people who received their first dose at eight medical institutions across the country since August.
Sixty-six percent felt fatigue, 51 percent had headaches, and 47 percent developed a fever of 37.5 degrees Celsius or higher.
The symptoms mostly occurred one or two days after the shot and were more common among young people.
This is the first time the government has released its survey on side effects of the AstraZeneca vaccine.
Professor Ito Suminobu at the medical school of Juntendo University heads the research team.
Ito says data from clinical trials showed that recipients of the AstraZeneca vaccine more likely experienced side effects after their first jab, while recipients of other vaccines developed symptoms more often after the second dose.
The professor says his team will continue to study side effects.
The health ministry says 28,997 people in Japan had received the AstraZeneca vaccine as of September 12, and no deaths had been reported.
One-thousand 190 people had been confirmed dead after receiving either the Pfizer-BioNTech or Moderna vaccines.
The ministry says the figure per million people was 17.2 for the Pfizer-BioNTech vaccine and 2.4 for the Moderna vaccine.
Officials say no causal link to the inoculation was established in any of those cases.


