研究:寮國蝙蝠冠狀病毒傳人結構極似COVID
中央社
10/15/2021
根據一份最新研究,在寮國蝙蝠身上的冠狀病毒出人意料地擅於感染人類細胞,顯示這類致命特徵確實可在實驗室以外的自然界演化而來,有助了解COVID-19疫情起源。
「紐約時報」指出,病毒專家對這一發現感到振奮。科學家2020年夏天前往寮國捕捉身上可能攜有COVID-19病原近親的蝙蝠,研究後得出相關發現。
科學家們趁夜深人靜時用網子和帆布所做的陷阱捕捉從洞穴飛出的蝙蝠,採集蝙蝠唾液、尿液和糞便樣本。確認蝙蝠糞便樣本內含有冠狀病毒後,科學家們便在高度安全的生物實驗室,以專門的防護裝備和空氣濾淨器展開研究。
研究人員發現,在寮國採集到的3種冠狀病毒很不尋常:它們表面有一個分子鉤,與這次引發COVID-19大流行、名為SARS-CoV-2的新型冠狀病毒身上的鉤極其相似。與新型冠狀病毒一樣,寮國蝙蝠身上冠狀病毒的鉤子讓它們能輕易附著於人體細胞。
談到寮國蝙蝠冠狀病毒的分子鉤是如何輕易感染人類時,帶領這項研究的巴黎「巴斯德研究所」(Pasteur Institute)病毒學家艾洛瓦(Marc Eloit)說:「它甚至比早期的新型冠狀病毒株還要好。」這份研究日前已於線上發布,但尚未發表於科學期刊。
有些專家認為,這些類SARS-CoV-2病毒很可能已不時感染人類,引發過輕微與幅度有限的傳播,但只要條件成熟,恐就能引起類似COVID-19那種大流行。
專家們表示,這些發現對有關COVID-19起源的激烈辯論具有重要意義。有些人堅信像SARS-CoV-2這般驚人感染人類細胞的能力不可能是自然進化而來,但這項新發現表明並非如此。
亞利桑那大學病毒學家沃洛比(Michael Worobey)說:「這真的能讓所有認為這種病毒肯定出於實驗室製造,或某種人為改造才能有如此傳染力的想法一槍斃命。」沃落比並未參與這項寮國蝙蝠冠狀病毒研究。

Tel: 551-580-4856 | Email: F.WINNIE.S@GMAIL.COM
诚招美国和加拿大法律服务代理
因公司发展需要,诚招美国和加拿大法律服务代理。
要求:
懂英语、或西班牙语、或法语。
能合法工作有社安号或工号。
无需改行, 可以兼职。
大学生和有销售经验优先考虑。
自雇生意公司发美国报税1099,加拿大T4A
有意了解详情, 请扫码加微信, 非诚勿扰!

研究:柬埔寨10年前采集蝙蝠病毒样本似新冠
中央社
9/20/2021

(中央社柬埔寨上丁20日綜合外電報導)研究人員發現,10年前在柬埔寨北部從蝙蝠身上採集的樣本和目前傳遍全球的新型冠狀病毒非常相似,因此再度前往採樣,希望更了解這種病毒的起源與演化。
路透社報導,2010年,研究人員在毗鄰寮國的上丁省(Stung Treng)從蹄鼻蝙蝠(horseshoe bats)身上採集了兩個樣本,保存在金邊的柬埔寨巴斯德研究所(Institut Pasteur)冰箱中。
去年檢測後發現,這些樣本與新型冠狀病毒有近親關係。全球迄今已有超過460萬人死於COVID-19(2019冠狀病毒疾病)。
由8名成員組成的柬埔寨巴斯德研究所團隊一週以來,一直在蝙蝠身上採集樣本,並記錄物種、性別、年齡與其他詳細資訊,類似研究也正在菲律賓進行。
田野調查協調員霍姆(Thavry Hoem)邊拿著網子捕捉蝙蝠邊告訴路透社:「我們希望這項研究成果可以幫助世界更了解COVID-19。」
蝙蝠等宿主物種通常不會表現出病原體症狀,但如果病原體傳染給人類或其他動物,可能帶來毀滅性後果。
柬埔寨巴斯德研究所病毒學部門主任韋斯納.楊(Veasna Duong)表示,研究所過去兩年已4度展開類似行程,希望找到這種病毒起源和演化的線索。
他說:「我們想知道病毒是否仍存在於當地…也想知道病毒如何演化。」
源自蝙蝠的致命病毒包括伊波拉病毒(Ebola)與其他冠狀病毒,像是嚴重急性呼吸道症候群(SARS)與中東呼吸症候群冠狀病毒(MERS)。
但韋斯納.楊說,由於人為干擾和破壞自然棲地,COVID-19帶來的毀滅性後果是人類咎由自取。
他表示:「如果我們試圖靠近野生動物,從野生動物身上接觸到病毒的機率就高於平常,病毒轉為感染人類的可能性也就更高。」
Cambodia Bat Researchers On Mission To Track Origin Of COVID-19
By Cindy Liu and Prak Chan Thul
9/20/2021
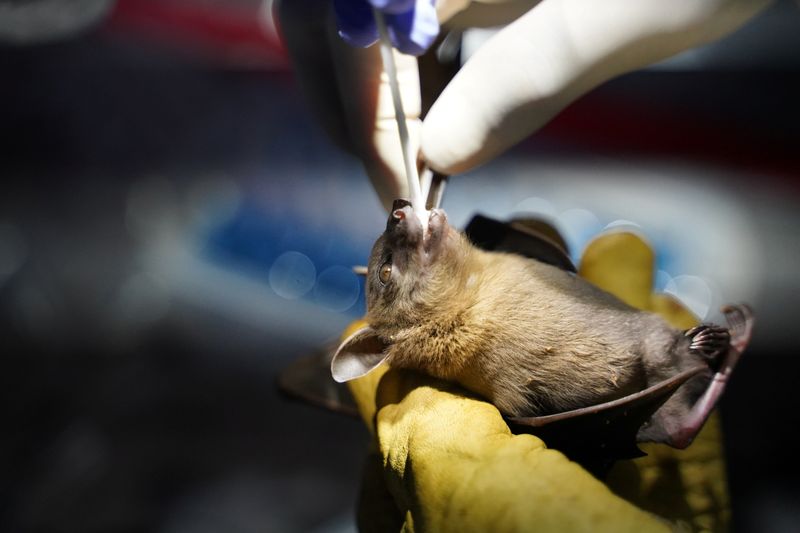
STUNG TRENG, Cambodia (Reuters) – Researchers are collecting samples from bats in northern Cambodia in a bid to understand the coronavirus pandemic, returning to a region where a very similar virus was found in the animals a decade ago.
Two samples from horseshoe bats were collected in 2010 in Stung Treng province near Laos and kept in freezers at the Institut Pasteur du Cambodge (IPC) in Phnom Penh.
Tests done on them last year revealed a close relative to the coronavirus that has killed more than 4.6 million people worldwide.
An eight-member IPC research team has been collecting samples from bats and logging their species, sex, age and other details for a week. Similar research https://reut.rs/3EsZXVO is going on in the Philippines.
“We hope that the result from this study can help the world to have a better understanding about COVID-19,” field coordinator Thavry Hoem told Reuters, as she held a net to catch bats.
Host species such as bats typically display no symptoms of pathogens, but these can be devastating if transmitted https://tmsnrt.rs/3lvfsE9 to humans or other animals.
Dr. Veasna Duong, Head of Virology at the IPC, said his institute had made four such trips in the past two years, hoping for clues about the origin and evolution of the bat-borne virus.
“We want to find out whether the virus is still there and … to know how the virus has evolved,” he told Reuters.
Deadly viruses originating from bats include Ebola and other coronaviruses such as Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).
But Veasna Duong said humans were responsible for the devastation caused by COVID-19, due to interference and destruction of natural habitats.
“If we try to be near wildlife, the chances of getting the virus carried by wildlife are more than normal. The chances of the virus transforming to infect humans are also more,” he said.
The French-funded project also aims to look at how the wildlife trade could be playing a part, said Julia Guillebaud, a research engineer at the IPC’s virology unit.
“(The project) aims to provide new knowledge on wild meat trade chains in Cambodia, document the diversity of betacoronaviruses circulating through these chains, and develop a flexible and integrated early-detection system of viral spill-over events,” Gillebaud said.
研究:老挝蝙蝠携带的病毒最接近冠病原始毒株
文 / 陈慧璋
9/18/2021

(早报讯)研究人员发现,栖息在老挝北部石灰岩洞穴里的蝙蝠携带的冠状病毒,与严重急性呼吸综合征冠状病毒2(SARS-CoV-2,即冠病原始毒株)具有共同的关键特征。
彭博社报道,这一发现让科学家对2019冠状病毒疾病(Covid-19)的起因再靠近一步。
法国巴斯德研究所和老挝大学的研究人员在数百只马蹄蝠中寻找与导致冠病相似的冠状病毒,并发现了三个紧密匹配的受体结合域。
这项论文报告周五(17日)刊登在《自然》期刊。研究人员在报告中指出,自然界中存在着与SARS-CoV-2密切相关的病毒,这些病毒来自某些蝙蝠种类包括几种中华菊头蝠(Rhinolophus)。中华菊头蝠又称马蹄蝠(horseshoe bat)。
这项研究为冠病大流行始于蝙蝠传播病毒的假设提供了支持。
9月14日发布的另一项研究显示,在中国南部和东南亚一些中华菊头蝠的密集栖息地,每天可能发生大约1000起此类感染病例。
巴斯德研究所病原体发现负责人兼论文合著者埃洛伊特(Marc Eloit)说,在老挝发现的三种病毒——BANAL-52、BANAL-103和BANAL-236,是“迄今为止最接近SARS-CoV-2的祖先病毒。”
埃洛伊特说:“这些病毒可能促成了SARS-CoV-2的起源,并可能在本质上构成未来直接传播给人类的风险。”
Bats in Laos caves found to carry coronaviruses that share key feature with Sars-CoV-2
9/18/2021
VIENTIANE (BLOOMBERG) – Bats dwelling in limestone caves in northern Laos were found to carry coronaviruses that share a key feature with Sars-CoV-2, moving scientists closer to pinpointing the cause of Covid-19.
Researchers at France’s Pasteur Institute and the University of Laos looked for viruses similar to the one that causes Covid-19 among hundreds of horseshoe bats.
They found three with closely matched receptor binding domains – the part of the coronavirus’ spike protein used to bind to human ACE-2, the enzyme it targets to cause an infection.
The finding, reported in a paper released on Friday (Sept 17) that is under consideration for publication by Nature journal, shows that viruses closely related to Sars-CoV-2 exist in nature, including in several Rhinolophus, or horseshoe bat, species.
The research supports the hypothesis that the pandemic began from a spillover of a bat-borne virus.
About 1,000 such infections may be occurring daily in southern China and South-east Asia in areas with dense populations of bats from the Rhinolophus genus, a study on Tuesday found.
The three viruses found in Laos, dubbed BANAL-52, BANAL-103, and BANAL-236, are “the closest ancestors of Sars-CoV-2 known to date”, said Dr Marc Eloit, head of pathogen discovery at the Pasteur Institute in Paris, and co-authors.
“These viruses may have contributed to Sars-CoV-2’s origin and may intrinsically pose a future risk of direct transmission to humans.”
The receptor binding domains of three Laos coronaviruses are closer to that of Sars-CoV-2 than to the RaTG13 virus identified in Rhinopholus affinis bats from the Mojiang mineshaft in Yunnan province, that was regarded as the pandemic strain’s closest match.
The BANAL-236 virus has an almost identical receptor binding domain to the pandemic virus, according to the paper.
“The receptor binding domain of Sars-CoV-2 looked unusual when it was first discovered because there were so few viruses to compare it to,” said Dr Edward Holmes, an evolutionary biologist at the University of Sydney, who was not involved in the research.
Tracing ancestors
“Now that we are sampling more from nature, we are starting to find these closely related bits of gene sequence,” Dr Holmes said in an e-mail on Saturday. “Eventually, with more sampling, the natural ancestry of the entire Sars-CoV-2 genome will be revealed.”
None of the bat viruses isolated in Laos harbours a so-called furin cleavage site in the spike that facilitates cell entry. It is a feature of the Sars-CoV-2 virus that has led some scientists to theorise that it was created in a laboratory.
No evidence supporting the lab-leak theory has emerged. Last month, the United States intelligence community ruled out the possibility that Sars-CoV-2 was developed by China as a biological weapon, but no consensus was reached on its origin.
The lack of furin cleavage may be explained by insufficient sampling in bats, or by acquisition of the furin cleavage site during chains of transmission in an alternate animal host, or during unreported circulation in humans in the early stages of the outbreak when the virus may have caused few symptoms, the authors said.
“Our results pinpoint the presence of new bat sarbecoviruses that seem to have the same potential for infecting humans as early strains of Sars-CoV-2,” they said.
Guano collectors
People who spend time in or close to caves, such as guano collectors, are particularly at risk of being exposed.
Further investigations are needed to assess if people exposed to bats have been infected by one of these viruses and whether they have antibodies that may provide protection against subsequent Sars-CoV-2 infections.
“This paper is really interesting and we need more research like it,” said Dr Maria Van Kerkhove, the World Health Organisation’s technical lead for Covid-19, in an e-mail.
The researchers studied 645 bats from 46 species captured on four sites – in Fueng and Meth districts of Vientiane province, and in Namor and Xay districts in Oudomxay province – between July last year and January this year.
The bats live in the limestone karstic terrain common to China, Laos, and Vietnam in the Indochina peninsula.
The paper highlights the diversity of Sars-CoV-2-like viruses present in bats in South-east Asia, Dr Holmes said.
“Continual sampling is the only way to understand the origins of this virus and it is important that more sampling is done throughout China as this remains the most likely place of origin,” he said.
“This study emphasises that bat coronaviruses that have the potential to infect humans readily exist in nature and could emerge at any point. This is the clear risk for the future.”



