联邦准了父母不准 42%家长不愿让孩子接种疫苗
世界新闻网
11/01/2021
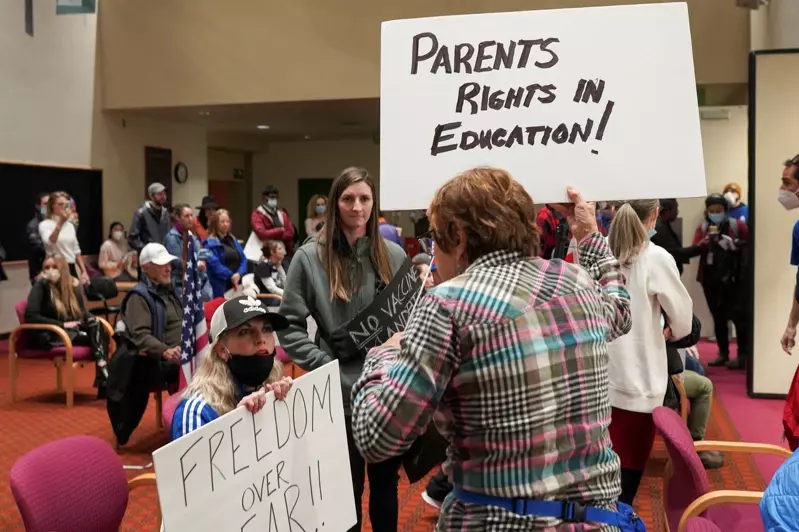
联邦食品暨药物管理局(FDA)已开放5至11岁的儿童接种新冠肺炎疫苗,让全美增加2800万人具备资格;虽然许多成人已完成接种,但仍不倾向让小孩接种;毕竟新冠肺炎对小孩造成的症况轻微,家长不让小孩接种可能引发未知的风险;如何让儿童接种,成为下一阶段最具挑战性的防疫任务。
辉瑞(Pfizer)疫苗日前已取得授权,低剂量注射不仅安全,且能给予小孩足够免疫力。常见的副作用,仍包括精神不佳、发烧和头痛。
联邦疾病防治中心(CDC)传染病专家表示,接下来假期旅游与聚会将更为频繁,小孩接种可能是改变疫情的关键;接种将让小孩能持续到学校上课,降低隔离的几率,并且防止将病毒传染给年长和高风险的成人。疫情爆发至今,全美已有近200万名5至11岁的儿童染疫,8300人住院;住院的儿童患者有三分之一被送进加护病房,至少170人死亡。

但研究显示,父母对于儿童接种的忧虑,在6月至9月间「显著增加」。最主要的因素,是对疫苗的不了解,包括是否有足够试验、副作用、保护力以及长期健康疑虑。
凯瑟家庭基金会(Kaiser Family Foundation)指出,只有近三分之一的家长,愿意立刻让小孩接种;另外三分之二为不愿意或反对。Axios与艾普索斯(Ipsos)的民调结果,也有42%家长强烈表示,不可能让小孩接种。
罗德岛州的家长艾琳‧高奇(Erin Gauch)的两个小孩虽然已接种,但仍非常担心副作用,尤其是发生在少数年轻男性身上的心肌炎。她说:「万一小孩出了什么事,我绝对没办法接受。」
德州州立大学医学人类学者艾蜜莉‧布朗森(Emily Brunson)研究接种选择,她表示对父母而言,为自己第一个孩子做接种的决定格外不容易,但现在甚至有父母表示,若未来学校强制学生接种,就要让小孩退学。
COVID shots are a go for children, but parents are reluctant to consent
By JAN HOFFMAN
11/01/2021

The Food and Drug Administration’s authorization of a COVID-19 vaccine for children ages 5-11 on Friday makes 28 million unvaccinated children in the United States suddenly eligible for the shot and offers the country an opportunity to make big inroads in its efforts to achieve broad immunity against the coronavirus.
But in a nation that has already struggled with COVID vaccine hesitancy, getting shots into those little arms may present health authorities with the toughest vaccination challenge yet.
Even many parents who are themselves vaccinated and approved the shot for their teenagers are churning over whether to give consent for their younger children, questioning if the risk of the unknowns of a new vaccine is worth it when most coronavirus cases in youngsters are mild.
In announcing its authorization of a lower-dose shot made by Pfizer and BioNTech for the age group, the FDA said clinical trial data showed the shot was safe and prompted strong immune responses in children. The most common side effects were fatigue, fever and headache.
Infectious disease experts say that with approaching holiday travel and family gatherings, widespread vaccination of younger children could help keep classes in person, reduce the likelihood of quarantines and lessen the risk of transmission to older, vulnerable adults — as well as protect the children from what has become the eighth-biggest killer in their age group, according to the Centers for Disease Control and Prevention. To date, nearly 2 million children ages 5-11 have been infected with the virus, and 8,300 have been hospitalized. One-third of those hospitalized were admitted to intensive care units, and at least 170 have died.
But a report this month from researchers at Northeastern, Harvard, Rutgers and Northwestern universities found that parental concerns around the COVID vaccination had increased “significantly” from June through September. Chief among them, researchers said, were the newness of the vaccine, whether it has been sufficiently tested, efficacy, side effects and long-term health consequences.

According to a survey released Thursday by Kaiser Family Foundation, scarcely 1 in 3 parents will permit their children in this newly eligible age group to be vaccinated immediately. Two-thirds were either reluctant or adamantly opposed. An Axios-Ipsos poll found that 42% of parents of these children said they were unlikely to have their children vaccinated.
Erin Gauch, of Middletown, Rhode Island, got herself and her two older children, ages 14 and 12, vaccinated this summer. But she is worried about the potential side effects of the shots for her son. One of those side effects is myocarditis, a weakening of the heart muscle, that has been reported in a very small number of teenage boys and young men after getting a COVID shot.
“I’m looking at a 9-year-old, and if I make a bad decision and he ends up with some debilitating side effects or lifelong adverse reaction, I don’t think I could live with that,” she said.
This vaccine dilemma occurs at a turbulent cultural moment for parents of young children, who are often judged harshly on social media for their decisions. The choice can appear freighted with political affiliation. A decision can signify, intentionally or not, compassion or disregard for others and a willingness to follow or ignore advice from their pediatrician.

“If we ultimately decide not to get my youngest vaccinated right now, I guess I’ll be subjected to mommy shaming, but I’ll just have to deal with it,” Gauch said.
Many parents, like Gauch, are focusing on some research that suggests the rare possibility that young men and boys will develop pericarditis, a weakening of the lining around the heart, and myocarditis, but the clinical trial data the FDA reviewed showed no cases in the 5-11 age group. Many experts say that the conditions usually improve quickly and that COVID presents far greater risk of severe myocarditis.
The Biden administration recently announced that the shots would be given predominantly by pediatricians, community health centers and children’s hospitals, plus pharmacies and schools, which will carry the burden of persuading parents.
But a Kaiser policy brief noted that schools and pharmacists in regions where COVID vaccination rates are low may be reluctant to participate. Access in rural areas and for working parents will be considerable challenges, the authors said, and they noted that achieving equity will also be a concern; more than half of those newly eligible are children of color.

After what is expected to be an initial rush of eager parents (as happened with adults and teenagers), pediatricians say they are bracing for conversations they anticipate to be among the thorniest they have ever had.
“I know parents are probably bombarded with misinformation about vaccines, even within their social circles: ‘My friend said this. My mother-in-law said that,’” said Dr. Katherine Williamson, a pediatrician in Orange County, California. “I’m hoping I can make a difference.”
The decision is particularly hard for parents to make on behalf of their first child, said Emily Brunson, a medical anthropologist at Texas State University who researches parent vaccination choices. Because vaccine decision-making is so personal and complicated, she said, many parents are likely to put it off.
Vic Sandrin, who works for a bicycle company in Vancouver, Washington, supports vaccines but cautiously. He, his wife and their 18-year-old got the COVID vaccine grudgingly, to travel for work and family visits.
For his 11-year-old twins, however, he is content to wait.
“I’m willing to take a chance on myself, and that made sense; I’m an adult,” Sandrin said. “But for kids who already have strong immune systems, I don’t know if there’s a reason to get them vaccinated, or at least not just quite yet.”
At heart, the decision is about which unknown — COVID or the vaccine — parents fear more. They may stack factors such as social routines, older relatives, school protocols and the likelihood of severe illness to confirm their intuitive bias about whether to allow their child to get the shot.
Gauch, a mechanical engineer, calculated each family member’s risk individually. She has asthma, so for her, the vaccine was a no-brainer. Her 14-year-old daughter got her first job this summer; getting vaccinated meant she would not have to wear a face mask at work. And her 12-year-old daughter saw that getting vaccinated could open up possibilities of being maskless in public. Done and done.
But not only does Gauch worry about side effects in her 9-year-old son, she said that getting vaccinated will not release him from following other COVID rules because his school insists on masks and social distancing.
“He is much less likely to get COVID if they’re taking all these precautions,” she said. “So I just don’t see the risk payoff of the vaccine.”
Parents who were predisposed not to vaccinate their child tended to dismiss the threat of serious illness from COVID as minuscule, saying that children who became seriously ill most likely had underlying conditions.
The argument that vaccinating children contributes to the community’s overall health does not get much traction, either. Parents’ paramount focus is the well-being of their own child. Although health officials contend an important reason to vaccinate is to protect the child, some parents said they believed that their healthy children would be injected with a novel vaccine largely to safeguard older adults, who had already lived full lives.
In interviews, some parents said that if the vaccine gained full approval for children (as the adult dose has) and schools required it, they would consider withdrawing their students. Dr. Cynthia Bader, a pediatrician in the Seattle area with an 8-year-old son, said that if her school district issued a vaccine mandate, she would clap her hands with joy but “then cringe at the idea of all the parents who will be coming to me seeking counseling for vaccine exemption forms.”
Parents are siloing themselves with like-minded friends, which reinforces their thinking.
“It used to be that more people with different opinions would mesh, but now I don’t think that is the case,” said Abby Cooper of Bergen County, New Jersey, who is eager to get her five children vaccinated.
But she has friends who refuse.
“Their kids are going to school with my kids and putting them at risk for no reason. It’s very upsetting,” Cooper said. “So, sadly, I’ve lost friends over this.”
Many parents worry that the tension will impact the children themselves. Some foresee having to set boundaries about unvaccinated playmates, especially if exposure to the virus could jeopardize someone else in the home.
Many parents will be difficult to persuade. The CDC and the American Academy of Pediatrics have published talking points for pediatricians and other proponents of the COVID vaccine for children.
Consensus: First, address the parents’ questions. But if they do not want to hear The Talk, do not force it.
Consensus: Fear tactics — generalized descriptions of children suffering in COVID wards — do not work.
Consensus: Emphasize the benefits of the COVID vaccine to the child in terms of emotional and physical well-being, including some semblance of pre-COVID social life. Invoke quarantines, remote learning.
Kim Cobb hopes that her family’s COVID ordeals will show others the benefits of vaccinating all eligible family members. She, her husband and their two older daughters, 14 and 12, got vaccinated quickly. But in August, her unvaccinated 10-year-old twins came down with COVID. Soon after, Cobb, a climate scientist at Georgia Tech, and her husband tested positive for breakthrough infections. Their two vaccinated children remained healthy.
The parents became miserably ill but did not require hospitalization, which they believe is because they were vaccinated.
All recovered, but Cobb and one twin have lingering respiratory distress.
“We’re in the third month post-infection, and we have to see pulmonologists, we have inhalers, we’re on medication, and we’re still having breathing difficulties,” Cobb said. “And this is not a kid who ever had respiratory symptoms.
“It was not foreseeable,” she continued. “If you could avoid it, you would.”
This story was originally published at nytimes.com. Read it here.
美国推迟批准青少年使用莫德纳疫苗
文 / 陈慧璋
11/01/2021
(早报讯)美国莫德纳公司说,美国药品监管当局推迟批准青少年使用其冠病疫苗,以便有更多时间评估青少年接种该疫苗后出现心肌炎的潜在风险。
法新社报道,莫德纳公司星期天(10月31日)发表声明说,美国食品与药物管理局(FDA)29日通知该公司,表示需要更多时间来评估对于接种莫德纳疫苗引发心肌炎风险的国际分析。
声明说,美国FDA针对莫德纳疫苗用于12至17岁者的评估工作可能会持续到2022年1月。
心肌炎和另一相关风险——心包炎此前被指与辉瑞疫苗及莫德纳疫苗有关联,且主要涉及青春期的男孩和年轻男性。
但根据美国疾病控制与预防中心(CDC)8月发表的一项研究报告,其风险在感染冠病之后要高得多。
莫德纳公司表示,美国CDC发现,接种采用信使核糖核酸(mRNA)新技术研发的冠病疫苗后,出现心肌炎的情况极为罕见,而且症状一般轻微。
辉瑞疫苗和莫德纳疫苗都是采用mRNA新技术的冠病疫苗。
Pfizer给5岁小孩打疫苗,故意用小针头避免心肌炎
By Carwash ForFree
10/26/2021
Pfizer很清楚它的mRNA疫苗的短期副作用,即“心脏损伤”,为了减少5到11岁小孩的心肌炎,Pfizer把mRNA剂量降到成人剂量的三分之一,更重要的是,它还故意使用“小针头”注射。为什么?
因为小针头注射,mRNA疫苗大多数会注射在浅表的肌肉里,进入血液的mRNA就会减少,杀死心肌细胞的量就减少,所以,导致小孩心肌炎的可能减少了(刚刚好勉强达到FDA通过的benefit/risk的最低要求)。如果使用正常大小的针头,心肌炎就会高一点,就达不到FDA的要求了。
Pfizer为了达到FDA的最低要求,可谓是颇费心机。不过,故意使用“小针头”更加证实其mRNA疫苗对小孩心脏的损伤很大!
成人试验过更小剂量,但是产生的抗体比较少。
成人试验过10微克的小剂量,但是产生的抗体,跟30微克的正常剂量比较,只有41%的抗体,所以,Pfizer最终决定,成人使用30微克的剂量(不是10微克)。但是现在看来,这个剂量对于成人来说,还是太高了,副作用太大,杀死的心肌细胞太多了。
Fauci说,因为疫情太紧急,没有时间试验很多个不同的剂量,没有达到最佳剂量。基本上就是千老们,拍了一下脑袋,就决定用30微克。
幸好,Pfizer比Moderna稍微好一点,Moderna的千老,拍了一下脑袋,注射100微克的剂量,剂量太大,导致现在Moderna的心肌炎太严重,被北欧的国家全部禁止了。今年年底,北欧的国家就会公布他们的所有真实数据,Moderna的心肌炎数据会极其难看。
Moderna意识到问题很严重,赶快把booster的剂量降低到50微克,但是已经太晚了。已经有太多的人,心脏被损伤得很严重,无法挽回了。希望他们未来十年安好。

Tel: 551-580-4856 | Email: F.WINNIE.S@GMAIL.COM
诚招美国和加拿大法律服务代理
因公司发展需要,诚招美国和加拿大法律服务代理。
要求:
懂英语、或西班牙语、或法语。
能合法工作有社安号或工号。
无需改行, 可以兼职。
大学生和有销售经验优先考虑。
自雇生意公司发美国报税1099,加拿大T4A
有意了解详情, 请扫码加微信, 非诚勿扰!
Moderna’s vaccine is the most effective, but Pfizer and J&J also protect well, CDC-led study says
By Maggie Fox
9/18/2021
(CNN) A head-to-head study of all three authorized coronavirus vaccines in the United States finds the Moderna vaccine is slightly more effective than Pfizer’s in real-life use in keeping people out of the hospital, and Johnson & Johnson’s Janssen vaccine comes in third, but still provides 71% protection.
Pfizer’s vaccine provided 88% protection against hospitalization, and Moderna’s was 93% effective.
The US Centers for Disease Control and Prevention led a nationwide study of vaccination involving more than 3,600 adults hospitalized for Covid-19 between March and August.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11- August 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the team wrote in the CDC’s weekly report on death and disease, the MMWR.
“Although these real-world data suggest some variation in levels of protection by vaccine, all FDA-approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization.”
They found that the biggest difference between the vaccine made by Moderna and Pfizer/BioNtech’s vaccine was driven by a decline that started about four months after people were fully vaccinated with Pfizer’s vaccine.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech versus 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the team wrote.
“Vaccine effectiveness for the Pfizer-BioNTech vaccine was 91% at 14 -120 days after receipt of the second vaccine dose but declined significantly to 77% at more than 120 days,” the team wrote.
Pfizer’s and Moderna’s vaccines both use genetic material called messenger RNA to deliver immunity, but they use differing doses and slightly different formulations. The Janssen vaccine uses an inactivated common cold virus called adenovirus — a viral vector — to carry genetic instructions into the body.
“A single dose of the Janssen viral vector vaccine had comparatively lower anti-SARS-CoV-2 antibody response and vaccine effectiveness against COVID-19 hospitalizations,” the team said. “Understanding differences in vaccine effectiveness by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All FDA-approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization.”
CDC worked with researchers across the country to study 3,689 patients at 21 hospitals in 18 states for the study. They also looked at antibodies in the blood of 100 healthy volunteers after they had been vaccinated with one of the three available vaccines.
“These real-world data suggest that the two-dose Moderna and Pfizer-BioNTech mRNA vaccine regimens provide more protection than does the one-dose Janssen viral vector vaccine regimen. Although the Janssen vaccine had lower observed vaccine effectiveness, one dose of Janssen vaccine still reduced risk for COVID-19-associated hospitalization by 71%,” they wrote.
The study had limitations. “This analysis did not consider children, immunocompromised adults, or vaccine effectiveness against COVID-19 that did not result in hospitalization,” the team wrote. Plus, the volunteers were only followed for 29 weeks — just over six months.
消息:美国可能在10月授权5-11岁儿童接种辉瑞疫苗
文 / 陈慧璋
9/10/2021
(早报讯)消息人士说,美国高级卫生官员相信,美国5至11岁儿童可在10月底前获准接种辉瑞-BioNTech冠病疫苗。
路透社报道,两名知情者周五(10日)透露,辉瑞制药公司将能在本月底前向美国食品与药物管理局(FDA)提供足够的相关临床试验数据,以申请紧急使用授权。
他们说,食药局预计需要三周时间评估辉瑞疫苗对5至11岁儿童是否安全有效并做出决定。
近几周,美国因德尔塔变种病毒肆虐导致冠病确诊病例大幅攀升,数以百万计的年幼儿童父母,特别是有学龄儿童的父母都殷切希望能为孩子加强防疫保护。
Schools grapple with thousands in isolation or quarantine as delta variant rages
Many schools that planned to forgo virtual and hybrid learning this year, in favor of in-person classes, are revisiting those plans as outbreaks require students to quarantine.
By Daniella Silva
9/05/2021

Schools that have opened their doors to students amid the spread of the highly contagious delta variant of the coronavirus already are grappling with how to best continue teaching students when hundreds and sometimes thousands of them are in quarantine.
As cases of Covid-19 started to wane over the spring and the early summer, many schools planned to forgo virtual and hybrid learning this year, in favor of in-person classes. But the lag in vaccination rates and the rapid spread of the delta variant have led districts to revisit those plans, with varying results.
Some schools have sent students home with packets of self-guided work to submit at the end of each day or when on-campus classes resume. Others have temporarily switched to virtual learning. And in some cases, schools have canceled classes altogether while campuses are closed or kids are in quarantine.

In Mississippi, officials last month said more than 20,000 students were in quarantine from exposure to the coronavirus after the first week of school.
The high quarantine numbers among students and staff members led at least 29 schools to “go virtual for a short time in order to interrupt transmission,” the state’s epidemiologist said.
Similarly, the Connally Independent School District in Texas, which opened to students Aug. 18, closed its schools for in-person classes Tuesday after two teachers died last week of Covid. The five schools in the suburban Waco district hastily switched to remote learning and are scheduled to reopen for in-person learning after Labor Day.
“If this pandemic has taught us one thing, it is the ability to change directions quickly,” Connally Elementary School Principal Eric Cantu wrote in a note to parents on the district’s website. “Going from Face to Face learning to remote learning is an example of this.”
More than 300 miles east, however, in Iraan, Texas, learning was put on hold for two weeks when schools closed because of a Covid outbreak in August, about a week into the school year.
Iraan-Sheffield Independent School District Superintendent Tracy Canter said in the letter to the school district’s community Aug. 16 that neither virtual nor remote learning would take place during the closure, but that teachers would be available by email.
She said schools would still meet the minimum 75,600 minutes of instructional time required by law, in part by turning two early-release days into full days of school.
“We know that this is difficult for everyone involved and we thank you for your support and patience during this unprecedented time,” Canter said in the letter.
School districts are confronting the dilemma of how to keep the school year going as the number of parents who want their children to go to in-person school drops.
Only 43 percent of public school parents and guardians said they wanted their children in a classroom full time this year after the Centers for Disease Control and Prevention on July 27 updated its health guidance to recognize the threat of the delta variant.
That was down from 58 percent of parents and guardians who said they wanted in-person instruction before July 27, according to a survey funded by the CDC through the Atlanta-based nonprofit CDC Foundation. It polled 1,448 public school parents and guardians July 23-Aug. 8.
Just more than half of the nation’s largest 100 school districts are planning to make a remote learning option available to all students, according to the University of Washington’s Center on Reinventing Public Education, while 92 will offer some type of remote instruction.
In Florida’s Hillsborough County Public Schools, thousands of students have been quarantined since the start of the school year in August.
Teachers are putting lessons and instructional material online for kids in quarantine and sending out electronic devices or providing physical copies of work based on student need, Jennifer Sparano, the district’s Covid coordinator, said.
She said work is done independently, with check-ins from teachers, but the district is looking into hiring teachers and support staff to be available online during the day to give children live access to a teacher if their own ones are not available because they are teaching in a classroom.
There were more than 7,380 reported Covid cases and nearly 9,270 students and staff in isolation or quarantine, as of Friday, according to a district dashboard. Last Friday, there were more than 5,270 reported Covid cases and nearly 12,000 students and staff in isolation or quarantine, according to the dashboard. The total district population is more than 240,000.
A symptom-free student could still be out of school for five to seven days, under the district’s quarantine procedures, though Sparano said said many students don’t typically miss five full days of school.
In Metro Nashville Public Schools in Tennessee, from Aug. 9 to Aug. 15, there were 95 staff who were isolated or quarantined, with 52 confirmed cases and 980 students were isolated or quarantined, with 207 confirmed cases. From Aug. 16 to Aug. 22, there were 143 staff isolated or quarantined, with 67 confirmed cases and 2,879 students isolated or quarantined, with 395 confirmed cases, according to Sean Braisted, the district’s executive officer of communications and community engagement.
Isolated or quarantined students can access their course information and assignments online and the district offers remote learning assistance to students, he said in an email. Parents must also verify a student participated in all instructional activities, he said.
In the Northeast, where many schools don’t reopen until after Labor Day, school districts are announcing return plans that include planning for continuing education in quarantine.
New York City, which houses the nation’s largest school system and where all public school teachers and staff must get vaccinated, is planning to try to limit the number of students who have to quarantine.
In elementary schools, a classroom will be required to quarantine for 10 calendar days in the event of a positive test in that class and students will continue to receive instruction while they quarantine. But in middle and high schools, students who are at least 12 years old, vaccinated and not showing symptoms will be allowed to continue to attend school in-person. Students who are at least 12 years old, vaccinated and showing symptoms will have to quarantine for 10 calendar days and will have access to remote learning while quarantining. Unvaccinated students will have to quarantine for 10 calendar days, continue learning remotely, and will be allowed to return with a negative result after the seventh day.
The city’s strategy is designed to avoid the disruption of major school closures, which would be necessary only in case of widespread transmission in the school, the New York City Department of Education said in a health and safety guide.
“Our commitment to parents is to minimize disruption this year, keep as much continuity as possible and make sure all those health and safety measures are in place,” New York City Mayor Bill de Blasio said.






