FDA NEWS RELEASE
FDA Approves First COVID-19 Vaccine
Approval Signifies Key Achievement for Public Health
For Immediate Release:August 23, 2021
Today, the U.S. Food and Drug Administration approved the first COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 disease in individuals 16 years of age and older. The vaccine also continues to be available under emergency use authorization (EUA), including for individuals 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals.
“The FDA’s approval of this vaccine is a milestone as we continue to battle the COVID-19 pandemic. While this and other vaccines have met the FDA’s rigorous, scientific standards for emergency use authorization, as the first FDA-approved COVID-19 vaccine, the public can be very confident that this vaccine meets the high standards for safety, effectiveness, and manufacturing quality the FDA requires of an approved product,” said Acting FDA Commissioner Janet Woodcock, M.D. “While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.”
Since Dec. 11, 2020, the Pfizer-BioNTech COVID-19 Vaccine has been available under EUA in individuals 16 years of age and older, and the authorization was expanded to include those 12 through 15 years of age on May 10, 2021. EUAs can be used by the FDA during public health emergencies to provide access to medical products that may be effective in preventing, diagnosing, or treating a disease, provided that the FDA determines that the known and potential benefits of a product, when used to prevent, diagnose, or treat the disease, outweigh the known and potential risks of the product.
FDA-approved vaccines undergo the agency’s standard process for reviewing the quality, safety and effectiveness of medical products. For all vaccines, the FDA evaluates data and information included in the manufacturer’s submission of a biologics license application (BLA). A BLA is a comprehensive document that is submitted to the agency providing very specific requirements. For Comirnaty, the BLA builds on the extensive data and information previously submitted that supported the EUA, such as preclinical and clinical data and information, as well as details of the manufacturing process, vaccine testing results to ensure vaccine quality, and inspections of the sites where the vaccine is made. The agency conducts its own analyses of the information in the BLA to make sure the vaccine is safe and effective and meets the FDA’s standards for approval.

Tel: 551-580-4856 | Email: F.WINNIE.S@GMAIL.COM
Comirnaty contains messenger RNA (mRNA), a kind of genetic material. The mRNA is used by the body to make a mimic of one of the proteins in the virus that causes COVID-19. The result of a person receiving this vaccine is that their immune system will ultimately react defensively to the virus that causes COVID-19. The mRNA in Comirnaty is only present in the body for a short time and is not incorporated into – nor does it alter – an individual’s genetic material. Comirnaty has the same formulation as the EUA vaccine and is administered as a series of two doses, three weeks apart.
“Our scientific and medical experts conducted an incredibly thorough and thoughtful evaluation of this vaccine. We evaluated scientific data and information included in hundreds of thousands of pages, conducted our own analyses of Comirnaty’s safety and effectiveness, and performed a detailed assessment of the manufacturing processes, including inspections of the manufacturing facilities,” said Peter Marks, M.D., Ph.D., director of FDA’s Center for Biologics Evaluation and Research. “We have not lost sight that the COVID-19 public health crisis continues in the U.S. and that the public is counting on safe and effective vaccines. The public and medical community can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U.S.”
FDA Evaluation of Safety and Effectiveness Data for Approval for 16 Years of Age and Older
The first EUA, issued Dec. 11, for the Pfizer-BioNTech COVID-19 Vaccine for individuals 16 years of age and older was based on safety and effectiveness data from a randomized, controlled, blinded ongoing clinical trial of thousands of individuals.
To support the FDA’s approval decision today, the FDA reviewed updated data from the clinical trial which supported the EUA and included a longer duration of follow-up in a larger clinical trial population.
Specifically, in the FDA’s review for approval, the agency analyzed effectiveness data from approximately 20,000 vaccine and 20,000 placebo recipients ages 16 and older who did not have evidence of the COVID-19 virus infection within a week of receiving the second dose. The safety of Comirnaty was evaluated in approximately 22,000 people who received the vaccine and 22,000 people who received a placebo 16 years of age and older.
Based on results from the clinical trial, the vaccine was 91% effective in preventing COVID-19 disease.
More than half of the clinical trial participants were followed for safety outcomes for at least four months after the second dose. Overall, approximately 12,000 recipients have been followed for at least 6 months.
The most commonly reported side effects by those clinical trial participants who received Comirnaty were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever. The vaccine is effective in preventing COVID-19 and potentially serious outcomes including hospitalization and death.
Additionally, the FDA conducted a rigorous evaluation of the post-authorization safety surveillance data pertaining to myocarditis and pericarditis following administration of the Pfizer-BioNTech COVID-19 Vaccine and has determined that the data demonstrate increased risks, particularly within the seven days following the second dose. The observed risk is higher among males under 40 years of age compared to females and older males. The observed risk is highest in males 12 through 17 years of age. Available data from short-term follow-up suggest that most individuals have had resolution of symptoms. However, some individuals required intensive care support. Information is not yet available about potential long-term health outcomes. The Comirnaty Prescribing Information includes a warning about these risks.
Ongoing Safety Monitoring
The FDA and Centers for Disease Control and Prevention have monitoring systems in place to ensure that any safety concerns continue to be identified and evaluated in a timely manner. In addition, the FDA is requiring the company to conduct postmarketing studies to further assess the risks of myocarditis and pericarditis following vaccination with Comirnaty. These studies will include an evaluation of long-term outcomes among individuals who develop myocarditis following vaccination with Comirnaty. In addition, although not FDA requirements, the company has committed to additional post-marketing safety studies, including conducting a pregnancy registry study to evaluate pregnancy and infant outcomes after receipt of Comirnaty during pregnancy.
The FDA granted this application Priority Review. The approval was granted to BioNTech Manufacturing GmbH.
Biden will announce vaccination requirement across federal government on Thursday
By Phil Mattingly and Jason Hoffman
7/27/2021
President Joe Biden will announce on Thursday a requirement that all federal employees and contractors be vaccinated against Covid-19, or be required to submit to regular testing and mitigation requirements, according to a source with direct knowledge of the matter.
The announcement will come in remarks where Biden is also expected to lay out a series of new steps, including incentives, in an attempt to spur new vaccinations as the Delta variant spreads rapidly throughout the country. It will also follow the decision by the Department of Veterans Affairs to require its frontline health care workers to be vaccinated over the course of the next two months.
Biden alluded to the looming announcement on Tuesday.
“That’s under consideration right now,” Biden said, when asked if he would impose a vaccination mandate on federal workers.
While the specifics are still being finalized, the source said, federal workers would be required to attest to their vaccination status or submit to regular testing. The source said the proposal will be roughly similar to what is being implemented in New York City. Additional requirements for the unvaccinated could be added as agencies push to vaccinate their employees.
Biden will not impose the requirement on the US military, despite his authority to do so, for the time being. He is, however, likely to outline how the Department of Defense may seek to approach the issue going forward, the source said.
Asked if he thinks the new revised guidance on masks from the US Centers for Disease Control and Prevention will lead to confusion for Americans, Biden cast blame on unvaccinated Americans, saying that if they had been vaccinated “we’d be in a very different world.”
“We have a pandemic because the unvaccinated and they’re sowing enormous confusion. And the more we learn about this virus and the Delta variation, the more we have to be worried and concerned. And there’s only one thing we know for sure, if those other hundred million people got vaccinated, we’d be in a very different world,” he said.
The administration’s decision to require vaccines for VA health workers provided a powerful signal that vaccine requirements could be necessary to convince the still-hesitant to get their shots.
Furthering the case for vaccine mandates, the administration is taking steps to spell out the legal grounds upon which American entities can require employees to get shots.
Justice Department lawyers have determined that federal law doesn’t prohibit public agencies and private businesses from requiring Covid-19 vaccines, even if the vaccines have only been authorized for emergency use, according to an opinion posted online Monday.
The opinion from the department’s Office of Legal Counsel — dated July 6, but released publicly Monday — paves the way for more federal agencies and businesses to require vaccinations following the Veterans Affairs announcement about front-line health workers.
In recent weeks, Justice Department officials have been weighing requests from private businesses and federal agencies seeking legal backing for policies aimed at encouraging vaccinations, according to people briefed on the matter.
The opinion marks a reversal from the previous administration. Last year, Attorney General William Barr used the Justice Department’s legal power to try to fight certain Covid restrictions, including joining some businesses that sought to overturn state mask mandates.
CNN’s Kevin Liptak contributed to this report.
Nearly 100 Positive COVID-19 Tests Linked to Illinois Summer Camp
6/29/2021
QUINCY, Ill. (WGEM/CNN) – Nearly 100 teenagers and staff tested positive for COVID-19 after attending a church camp in rural Illinois.
‘The Crossing Camp’ was held in mid-June in Rushville. While majority of the campers and staff were eligible for the vaccine, Illinois Department of Health officials said few had been inoculated.
The health department said the camp did not require masks indoor. At least one young adult was hospitalized as a result of the outbreak.
The Delta variant appears to have tripled to 31% of all US coronavirus cases in just 11 days. It’s more dangerous than other variants and could imperil the country’s recovery.
By Marianne Guenot
6/21/2021
As of Wednesday, the Delta coronavirus variant may have been responsible for 31% of all coronavirus cases in the US, according to an estimate by the Financial Times.
Previous data from the Centers for Disease Control and Prevention put that share of cases at about 10% as of June 5 and 2.7% on May 22.
The Financial Times estimate, based on available sequencing data, would mean the share of Delta-variant infections in the US tripled in just 11 days.
The CDC has not yet released data on the rate of Delta-variant cases after June 5 in the US.
Dr. Rochelle Walensky, the director of the CDC, warned last week that the Delta variant could soon become dominant in the US.
And on Sunday, the former Food and Drug Administration chief Scott Gottlieb warned that the Delta variant could lead to a surge of cases in the fall even if 75% of eligible Americans were vaccinated by then.
The variant, which was first identified in India and is also called B.1.617.2, appears to be 60% more transmissible than the variant that is now still dominant in the US, the Alpha variant.
Data from the UK, where the Delta variant is thought to make up about 90% of current coronavirus cases, also suggests that the risk of hospitalization is higher for unvaccinated people with this variant.
The Delta variant also appears to more likely to evade the protection given by partial vaccinations.
Research suggests a single shot of the Pfizer-BioNTech or Oxford University-AstraZeneca coronavirus vaccines may give just 33% protection against symptomatic COVID-19 cases with the Delta variant, compared with at least 88% for other variants.
Two doses of the Pfizer-BioNTech or Oxford-AstraZeneca shot have been found to be protective against hospitalization from the variant, however.
It is not clear what protection other vaccines give against this variant.
On Friday, President Joe Biden warned that the Delta variant was “particularly dangerous for young people,” who are less likely to be vaccinated than older adults and are more socially active.
He also urged all Americans to get second doses of vaccines that require them.
As of Monday, 45% of Americans were fully vaccinated and 53% had received one dose, according to data from the CDC. The pace of vaccination, however, has fallen in the past two months.
Six Spaces Home Staging

Contact: Hongliang Zhang
Tel: 571-474-8885
Email: zhl19740122@gmail.com
打完疫苗后却确诊 专家:疫苗并不会马上出现保护作用
文 / 潘万莉
12/30/2020
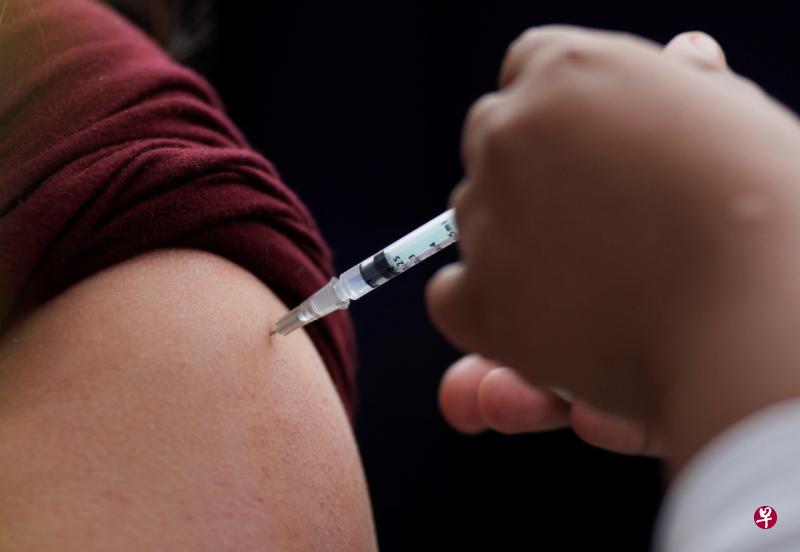
(早报讯)美国一名急诊科护士18日接种冠病疫苗后,仍旧在八天后确诊。传染病专家表示,疫苗并不会马上出现保护作用,至少得等10至14天后才有效;另外,冠状病毒的潜伏期较长,护士也可能在注射前就染疫。
根据报道,美国加州圣地牙哥一名45岁急诊室护士马修在18日接种了辉瑞的冠病疫苗,当时出现的副作用仅有手臂肌肉酸痛,没想到他却在24日出现发冷、全身肌肉疼痛和疲劳等症状,最后在26日经检测后发现确诊冠病。
圣地牙哥家庭健康中心传染病专家拉莫斯表示,这种状况其实并不意外,因为疫苗接种后,大约还需要10至14天才会出现防护效果,且还需要注射第二剂疫苗,“第一次注射的保护力约在50%,第二次则会提高到95%。”
拉莫斯说,这一切都代表着,尽管疫苗是一切结束的开始,但还是必须坚持基本的公卫守则,“总而言之,戴口罩、洗手并保持社交距离才是最重要的防疫措施。”
福奇:冠病早期治疗或是走向疫苗的桥梁
文 / 林煇智
9/27/2020

(早报讯)美国国家过敏和传染病研究所所长安福奇称,阻止2019冠状病毒疾病(COVID-19)在体内传播的单克隆抗体(monoclonal antibody),是疫苗面市前避免病毒导致严重疾病的策略之一。
彭博社报道,福奇表示,当局在研究把基于抗体的药物,以及其他来自康复患者的血液制品和抗病毒药作为早期治疗方法,而其目的是为了防止患者出现严重肺损伤。
福奇说:“我们现在非常重视早期感染的治疗或预防感染。那是通往疫苗的桥梁。”
福奇说,在美国针对SARS-CoV-2的免疫可能会于11月或12月开始,不过可能至少要到2021年第三季度才能让大多数美国人得到保护,以大大减轻这种大流行病毒的威胁。
福奇说,美国在12月份可能生产1亿剂疫苗,而美国旗下的六家药厂公司打算在明年4月前生产7亿剂。




